
The mass of hydrogen is thus 0.4667* 1 = 0.4667 The number of moles here is thus 4.2/18 = 0.2333īut there are 2 atoms of hydrogen in 1 mole of water and thus, the number of moles of hydrogen is 2 * 0.2333 = 0.4667 moles To get the number of moles of water, we need to divide the mass of water by its molar mass. The mass of carbon in the compound is thus 12 * 0.2341= 2.8091įrom the number of moles of water, we can get the number of moles of hydrogen. The mass of carbon in the compound is simply the number of moles multiplied by the atomic mass unit. Since there is only one carbon atom in CO2, the number of moles of carbon is same as above The number of moles of carbon iv oxide is 10.3/44 = 0.2341 The molar mass of carbon iv oxide is 44g/mol We first divide the mass by the molar mass of carbon iv oxide. #"For H: " (1.5765color(red)(cancel(color(black)("moles"))))/(0.78868color(red)(cancel(color(black)("moles")))) = 1.We can get the answer through calculations as follows.įrom the mass of carbon iv oxide produced, we can get the number of moles of carbon produced. #0.78868color(red)(cancel(color(black)("moles CO"_2))) * "1 mole C"/(1color(red)(cancel(color(black)("mole CO"_2)))) = "0.78868 moles C"#įinally, to find the mole ratio that exists between carbon and hydrogen in the hydrocarbon, divide these values by the smallest one SInce every mole of carbon dioxide contains 1 mole of carbon and 2 moles of oxygen, it follows that the reaction also produced Now, you know that every mole of water contains 2 moles of hydrogen and 1 mole of oxygen, which means that the reaction produced
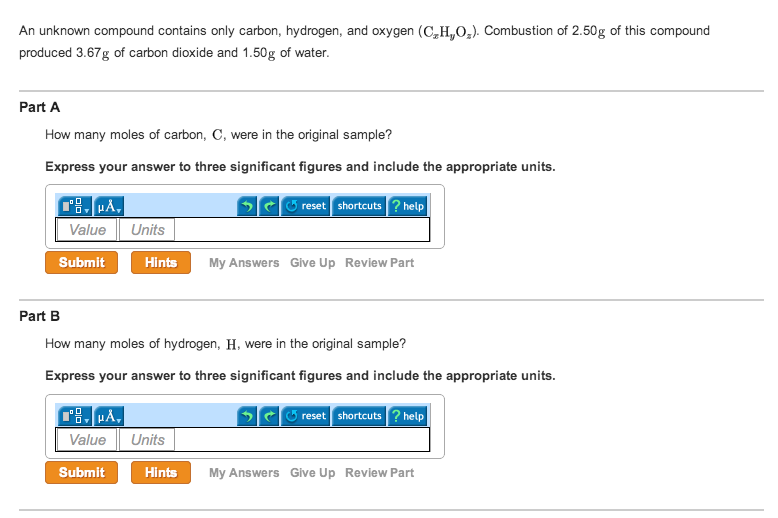

This means that you can use the number of moles of water and carbon dioxide, respectively, to determine how many moles of carbon and of hydrogen were originally present in the hdyrocarbon. Likewise, all the hydrogen that was initially a part of the hydrocarbon is now a part of the water. This tells you that all the carbon that was initially a part of the hydrocarbon will now be part of the carbon dioxide.

Notice that the products of this combustion reaction are carbon dioxide, #"CO"_2#, and water, #"H"_2"O"#. The key here is to realize that you're dealing with a hydrocarbon, that is, a compound that contains only carbon and hydrogen.


 0 kommentar(er)
0 kommentar(er)
